|
A simplified diagram (Figure 1) outlines polysaccharide breakdown and the main routes of carbohydrate fermentation in the human large intestine. Bacteria and, to a lesser extent, archaea contribute to processes. Two distinct cross-feeding mechanisms operate in the gastrointestinal tract: one due to the consumption of fermentation end-products (lactate, acetate, succinate) and the other due to cross-feeding of partial breakdown products from complex substrates (Falony et al., 2006; Belenguer et al., 2007; Reichardt et al., 2014). Both mechanisms contribute to the production of butyrate and propionate. 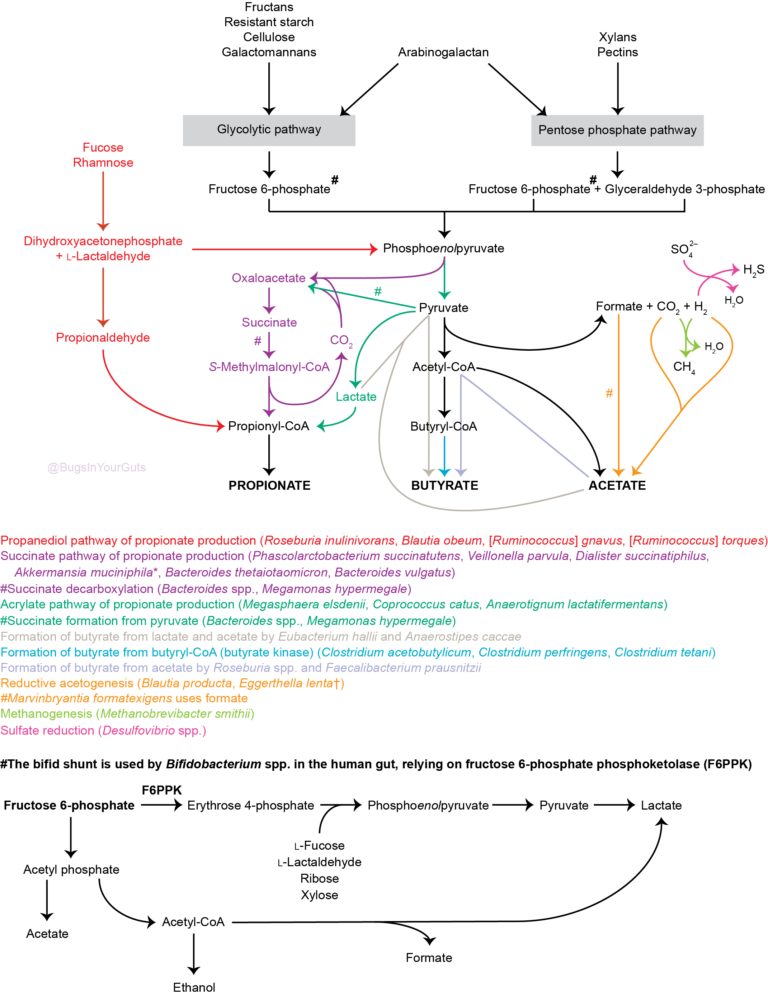 Figure 1. Summary of short-chain fatty acid production by the human gut microbiota. Updated from Hoyles & Wallace (2010) to include propionate formation (Reichardt et al., 2014) and the bifid shunt, which is restricted to Bifidobacterium spp. in the human gut via the action of fructose 6-phosphate phosphoketolase (Pokusaeva et al., 2011). *Akkermansia muciniphila is thought to be produce propionate via the succinate pathway. †Species predicted from sequence analyses to be capable of reductive acetogenesis (Ohashi et al., 2007; Hylemon et al., 2018). References Belenguer, A., Duncan, S. H., Holtrop, G., Anderson, S. E., Lobley, G. E. & Flint, H. J. (2007). Impact of pH on lactate formation and utilization by human fecal microbial communities. Appl Environ Microbiol 73, 6526–6533. Falony, G., Vlachou, A., Verbrugghe, K. & De, Vuyst, L. (2006). Cross-feeding between Bifidobacterium longum BB536 and acetate-converting, butyrate-producing colon bacteria during growth on oligofructose. Appl Environ Microbiol 72, 7835–7841. Hoyles, L. & Wallace, R. J. (2010). Gastrointestinal tract: intestinal fatty acid metabolism and implications for health. In Handbook of Hydrocarbon and Lipid Microbiology, pp. 3119–3132. Springer, Berlin. Hylemon, P. B., Harris, S. C. & Ridlon, J. M. (2018). Metabolism of hydrogen gases and bile acids in the gut microbiome. FEBS Lett doi:10.1002/1873-3468.13064. Ohashi, Y., Igarashi, T., Kumazawa, F. & Fujisawa, T. (2007). Analysis of acetogenic bacteria in human feces with formyltetrahydrofolate synthetase sequences. Biosci Microflora 26, 37–40. Pokusaeva, K., Fitzgerald, G. F. & van Sinderen, D. (2011). Carbohydrate metabolism in bifidobacteria. Genes Nutr 6, 285–306. Reichardt, N., Duncan, S. H., Young, P., Belenguer, A., McWilliam Leitch, C., Scott, K. P., Flint, H. J. & Louis, P. (2014). Phylogenetic distribution of three pathways for propionate production within the human gut microbiota. ISME J 8, 1323–1335. Like bacteria, archaea are prokaryotes (micro-organisms with neither a nucleus with a distinct membrane nor specialized organelles). Although they resemble bacteria morphologically, archaea have genes and metabolic pathways similar to those of eukaryotes (organisms whose cells have a distinct nucleus) and their cell membranes have high levels of glycerol-ether lipids, whereas cell membranes of bacteria and eukaryotes comprise mostly glycerol-ester lipids (Dridi et al., 2011). Because of their glycerol-ether lipids, archaeal cells are more difficult to break open than those of bacteria and, consequently, are likely to be under-represented in many DNA-based microbiota/microbiome studies (Dridi et al., 2009). There are five phyla of archaea (Euryarchaeota, Crenarchaeota, Nanoarchaeota, Xenarchaeota/Korachaeota, Thaumarchaeota), but only representatives of the Euryarchaeota are frequently found in the human gut microbiota, with methanogenic archaea predominating in this environment. The archaeal component of the gut microbiota is sometimes referred to as the archaeome. Until recently it was thought all archaea of the human gut microbiota were obligate anaerobes, but it is now possible to isolate some archaea aerobically from gut samples if they are grown with an external hydrogen (H2) source or in high salt conditions (Lagier et al., 2016). In addition to the methanogenic and halophilic archaea mentioned below, molecular studies have suggested the presence of members of the orders Methanosarcinales, Thermoplasmatales, Methanomicrobiales and Nitrososphaerales in the human gut microbiota, but none of these micro-organisms has been isolated (Dridi et al., 2011; Gaci et al., 2014). In many instances isolation and cultivation of archaea is complicated by their fastidious atmospheric requirements [e.g. 20 % CO2, 80 % H2, 1–25 bar (14.5–36 psi) for some of the methanogenic archaea] (Dridi et al., 2011). Methanogens Methanogens are archaea that generate methane (CH4) from a limited number of simple substrates [e.g. carbon dioxide (CO2); H2; formate (CHOO–); trimethylamine (TMA; N(CH3)3); methanol (CH3OH)] produced when dietary substrates and other organic matter are broken down by hydrolytic and fermentative microbes in the gut (Gaci et al., 2014; Figure 1). These simple substrates are considered the ‘end products’ of microbial catabolism. Between 30 and 40 % of H2 and CH4 produced in the intestine is absorbed by the colonic mucosa and taken up into the bloodstream, with the rest expelled from the body through belching or flatulence (Gibson et al., 1993; Carbonero et al., 2012; Figure 1). It has been suggested humans acquire methanogenic archaea via environmental contamination; once these microbes find favourable physicochemical and nutrient conditions in the gut, they colonize the intestine and become established members of the gut microbiota (Dridi et al., 2011). Breath tests suggest between a third and two-thirds of healthy adults produce CH4 (Levitt et al., 2006), while sensitive molecular-based studies show most adults carry methanogenic archaea in their intestine (>80–96 %: Mihajlovski et al., 2010; Dridi et al., 2009; Dridi et al., 2012a; Vanderhaeghen et al., 2015). Abundance of methanogenic archaea increases from the ascending (proximal) to the descending (distal) colon (0.003 % to 11 % of total gut prokaryotes; Dridi et al., 2011), and increases from childhood into adulthood and old age (Mihajlovski et al., 2010). The hydrogenotrophic (H2-utilizing) archaea belonging to the order Methanobacteriales (Methanibrevibacter, Methanosphaera) are the most frequently detected (89 % and 65 % of adults and children, respectively) and most abundant methanogens in human faeces (0.52 % and 0.15 % of the total faecal microbiota of adults and children, respectively) (Vanderhaeghen et al., 2015). Hydrogenotrophic methanogens use H2 for the reduction of CO2 and methyl-compounds. By removing H2 from the gastrointestinal tract, these archaea optimize fermentation in this environment and modify the metabolic pathways of fermentative bacteria (Gaci et al., 2014). Reduction of CO2 using H2 efficiently reduces the gas partial pressure in the colon (4 H2 + CO2 → CH4 + 2 H2O). The methylotrophic (one-carbon-compound-utilizing) members of the order Methanomassiliicoccales (Methanomassiliicoccus) are present in 50 % of adults, with prevalence seemingly increasing from infancy to adulthood (Dridi et al., 2012a; Vanderhaeghen et al., 2015). 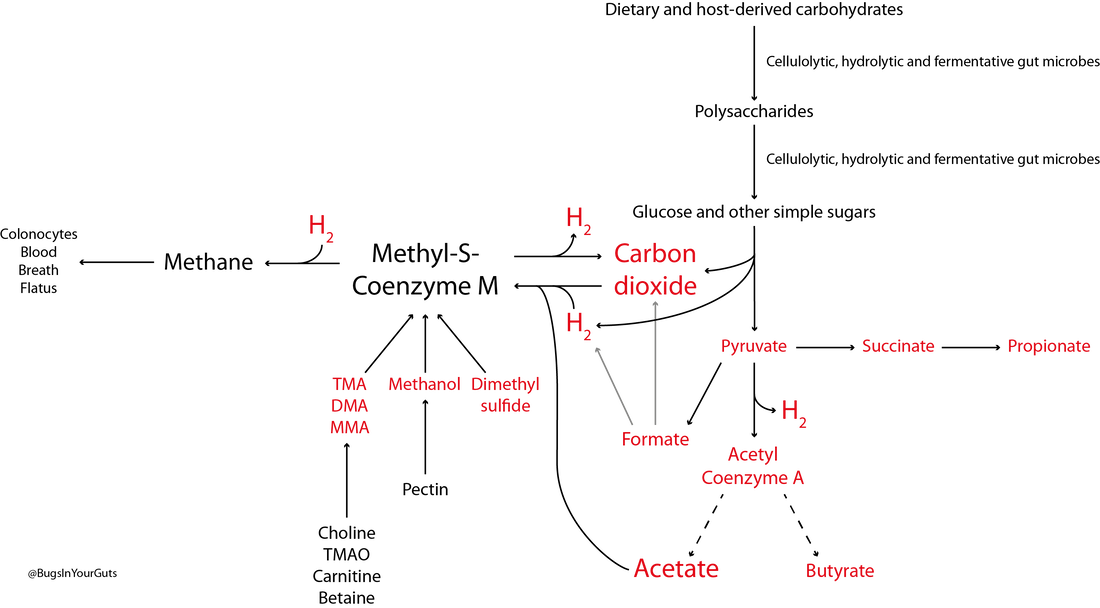 Figure 1. Microbial fermentation and hydrolysis of dietary substrates [predominantly polysaccharides (10–60 g/day) and proteins (6–18 g/day)] in the gastrointestinal tract leads to the release of end-products. Methanogens, therefore, have a terminal position in microbial trophic chains. The fate of carbohydrates, methylamines, methanol and dimethyl sulfide are shown in the figure. Broken arrows, incomplete pathways shown; full details can be found in the references cited below. In addition to those shown in red, ethanol, lactate, amino acids, ammonia, sulfate, phenolic compounds, indoles and branched-chain fatty acids are end-products of microbial catabolism released into the intestinal lumen. Methanobrevibacter smithii uses carbon dioxide (CO2) (or formate) and hydrogen (H2) to produce methane. Methanosphaera stadtmanae, members of the order Methanomassiliicoccales and possibly Methanobrevibacter smithii use methanol and H2 to produce methane. Members of the order Methanomassiliicoccales use methylamines (TMA, trimethylamine; DMA, dimethylamine; MMA, monomethylamine) and H2 to produce methane. Acetate to methane, aceticlastic methanogenesis. Methylamines, methanol and dimethyl sulfide to methane, methylotrophic methanogenesis. CO2 to methane: hydrogenotrophic methanogenesis. Information taken from Borrel et al. (2013a), Carbonero et al. (2012), Dridi et al. (2011), Gaci et al. (2014) and Chaudhary et al. (2015). Methanobrevibacter smithii Methanobrevibacter smithii is the most abundant archaeon in the human gut, and is detected in almost all adults (Mihajlovski et al., 2010). It can colonize the gut from the caecum to the rectum (Gaci et al., 2014). Cultivation work has shown it represents between 0.001 and 13 % of total anaerobes in human faeces (Miller & Wolin, 1982). Using a protocol optimized for the extraction and detection of archaeal DNA in faeces, it was shown that 95.5 % of individuals carry Methanobrevibacter smithii (Dridi et al., 2009). The archaeon has not been detected in the faeces of newborns (Mihajlovski et al., 2010), but has been detected in the faeces of children as young as 2 weeks of age (Dridi et al., 2009). It reduces CO2 to CH4 with H2 as the primary electron donor, and can use formate directly as a substrate to produce CH4 (Jones et al., 1987; Chaudhary et al., 2015; Vanderhaeghen et al., 2015). It can also use ammonium (NH4+) and ammonia (NH3) as sources of nitrogen (N) and consume ethanol (CH3CH2OH) present in the intestine, contributing to non-methanogenic removal of bacterial end-products of fermentation (Samuel et al., 2007; Hansen et al., 2011). The cell surface of Methanobrevibacter smithii is covered with carbohydrates (glycans) that mimic those found in the intestinal mucosa, facilitating its colonization of the intestine (Samuel et al., 2007). Methanobrevibacter smithii encodes a bile salt hydrolase that can degrade both tauro and glycol conjugated bile acids, thereby contributing to bile acid modification in the human gut (Jones et al., 2008). Methanomassiliicoccus luminyensis Methanomassiliicoccus luminyensis is currently the only cultivated member of the genus Methanomassiliicoccus. It was first isolated from human faeces on methanol and H2 (Dridi et al., 2012b). This methylotrophic archaeon has an energy metabolism distinct from other cultured methanogens: it can use methanol or TMA as an electron acceptor (and may be able to use dimethyl sulfide) to produce CH4 (Dridi et al., 2012b; Brugère et al., 2014). The ability of of Methanomassiliicoccus luminyensis to remove TMA from the gut has potential health benefits to humans, leading to the proposed use of archaebiotics in individuals with fish odour syndrome and those at risk of cardiovascular disease (Brugère et al., 2014). ‘Candidatus Methanomassiliicoccus intestinalis’ and ‘Candidatus Methanomethylophilus alvus’, related to Methanomassiliicoccus luminyensis, have been enriched from human faeces using methanol; both species are predicted to use methylamines to produce CH4. Although the draft genome sequences of these archaea are available, neither has been successfully isolated and preserved, hence their Candidatus status (Borrel et al., 2012; Borrel et al., 2013b). In addition to the human gut microbiota, 16S rRNA gene and/or mcrA (methyl:coenzyme M reductase, a functional marker gene of methanogens) sequences of members of the order Methanomassiliicoccales have been found in the gastrointestinal tract of the elephant, giant tortoise, tortoise, rat, cattle, sheep, yak, reindeer and wallaby, and the human oral cavity (Söllinger et al., 2016). Methanosphaera stadtmanae Methanosphaera stadtmanae is detected in up to a third of western adults, with abundance appearing to increase from adulthood into old age (Dridi et al., 2009; Mihajlovski et al., 2010). It only produces CH4 by reducing methanol (derived from microbial degradation of pectin) in the presence of H2, and needs acetate (CH3COOH) and CO2 as carbon sources (Fricke et al., 2006). Growth of Methanosphaera stadtmanae is enhanced in the presence of tungsten (Dridi et al., 2012c). In vitro, Methanosphaera stadtmanae and Methanobrevibacter smithii have been shown to form biofilms on various substrates; however, it is not know if it they contribute to mucosa-associated biofilms in the human gut (Bang et al., 2014a). In addition, both archaea have been shown to stimulate dendritic cells and peripheral blood mononuclear cells in vitro, suggesting they may be specifically recognized by the human innate immune system, though this has not been demonstrated in vivo (Bang et al., 2014b; Blais Lecours et al., 2014). Halophilic archaea As their name implies, halophiles are salt-loving organisms able to thrive in high salt concentrations (slight halophiles, 0.3–0.8 M NaCl; moderate halophiles, 0.8–3.4 M NaCl; extreme halophiles, 3.4–5.1 M NaCl). The human gut is not a salty environment (135–145 mM sodium), but moderatey halophilic, aerobic archaea belonging to the order Halobacteriales have been isolated in small numbers from intestinal mucosal samples taken from patients with inflammatory bowel disease (Oxley et al., 2010). More recently, the halophilic archaea Haloferax alexandrines and ‘Haloferax massiliensis‘ have been isolated from the human gut, but their role(s) and abundance in this environment are not yet known (Khelaifia & Raoult, 2016; Lagier et al., 2016). References Bang, C., Ehlers, C., Orell, A., Prasse, D., Spinner, M., Gorb, S. N., Albers, S. V. & Schmitz, R. A. (2014a). Biofilm formation of mucosa-associated methanoarchaeal strains. Front Microbiol 5, 353. Bang, C., Weidenbach, K., Gutsmann, T., Heine, H. & Schmitz, R. A. (2014b). The intestinal archaea Methanosphaera stadtmanae and Methanobrevibacter smithii activate human dendritic cells. PLoS One 9, e99411. Blais Lecours, P., Marsolais, D., Cormier, Y., Berberi, M., Haché, C., Bourdages, R. & Duchaine, C. (2014). Increased prevalence of Methanosphaera stadtmanae in inflammatory bowel diseases. PLoS One 9, e87734. Borrel, G., Harris, H. M., Tottey, W., Mihajlovski, A., Parisot, N., Peyretaillade, E., Peyret, P., Gribaldo, S., O’Toole, P. W. & Brugère, J. F. (2012). Genome sequence of “Candidatus Methanomethylophilus alvus” Mx1201, a methanogenic archaeon from the human gut belonging to a seventh order of methanogens. J Bacteriol 194, 6944-6945. Borrel, G., O’Toole, P. W., Harris, H. M., Peyret, P., Brugère, J. F. & Gribaldo, S. (2013a). Phylogenomic data support a seventh order of methylotrophic methanogens and provide insights into the evolution of methanogenesis. Genome Biol Evol 5, 1769-1780. Borrel, G., Harris, H. M., Parisot, N., Gaci, N., Tottey, W., Mihajlovski, A., Deane, J., Gribaldo, S., Bardot, O., Peyretaillade, E., Peyret, P., O’Toole, P. W. & Brugère, J. F. (2013b). Genome sequence of “Candidatus Methanomassiliicoccus intestinalis” Issoire-Mx1, a third Thermoplasmatales-related methanogenic archaeon from human feces. Genome Announc 1, pii:e00453-13. Brugère, J. F., Borrel, G., Gaci, N., Tottey, W., O’Toole, P. W. & Malpuech-Brugère, C. (2014). Archaebiotics: proposed therapeutic use of archaea to prevent trimethylaminuria and cardiovascular disease. Gut Microbes 5, 5-10. Carbonero, F., Benefiel, A. C. & Gaskins, H. R. (2012). Contributions of the microbial hydrogen economy to colonic homeostasis. Nat Rev Gastroenterol Hepatol 9, 504-518. Chaudhary, P. P., Gaci, N., Borrel, G., O’Toole, P. W. & Brugère, J. F. (2015). Molecular methods for studying methanogens of the human gastrointestinal tract: current status and future directions. Appl Microbiol Biotechnol 99, 5801-5815. Dridi, B., Henry, M., El Khéchine, A., Raoult, D. & Drancourt, M. (2009). High prevalence of Methanobrevibacter smithii and Methanosphaera stadtmanae detected in the human gut using an improved DNA detection protocol. PLoS One 4, e7063. Dridi, B., Raoult, D. & Drancourt, M. (2011). Archaea as emerging organisms in complex human microbiomes. Anaerobe 17, 56-63. Dridi, B., Henry, M., Richet, H., Raoult, D. & Drancourt, M. (2012a). Age-related prevalence of Methanomassiliicoccus luminyensis in the human gut microbiome. APMIS 120, 773-777. Dridi, B., Fardeau, M. L., Ollivier, B., Raoult, D. & Drancourt, M. (2012b). Methanomassiliicoccus luminyensis gen. nov., sp. nov., a methanogenic archaeon isolated from human faeces. Int J Syst Evol Microbiol 62, 1902-1907. Dridi, B., Khelaifia, S., Fardeau, M. L., Ollivier, B. & Drancourt, M. (2012c). Tungsten-enhanced growth of Methanosphaera stadtmanae. BMC Res Notes 5, 238. Fricke, W. F., Seedorf, H., Henne, A., Krüer, M., Liesegang, H., Hedderich, R., Gottschalk, G. & Thauer, R. K. (2006). The genome sequence of Methanosphaera stadtmanae reveals why this human intestinal archaeon is restricted to methanol and H2 for methane formation and ATP synthesis. J Bacteriol 188, 642-658. Gaci, N., Borrel, G., Tottey, W., O’Toole, P. W. & Brugère, J. F. (2014). Archaea and the human gut: new beginning of an old story. World J Gastroenterol 20, 16062-16078. Gibson, G. R., Macfarlane, G. T. & Cummings, J. H. (1993). Sulphate reducing bacteria and hydrogen metabolism in the human large intestine. Gut 34, 437-439. Hansen, E. E., Lozupone, C. A., Rey, F. E., Wu, M., Guruge, J. L., Narra, A., Goodfellow, J., Zaneveld, J. R., McDonald, D. T., Goodrich, J. A., Heath, A. C., Knight, R. & Gordon, J. I. (2011). Pan-genome of the dominant human gut-associated archaeon, Methanobrevibacter smithii, studied in twins. Proc Natl Acad Sci U S A 108 Suppl 1, 4599-4606. Jones, W. J., Nagle, D. P. Jr & Whitman, W. B. (1987). Methanogens and the diversity of archaebacteria. Microbiol Rev 51, 135-177. Jones, B. V., Begley, M., Hill, C., Gahan, C. G. & Marchesi, J. R. (2008). Functional and comparative metagenomic analysis of bile salt hydrolase activity in the human gut microbiome. Proc Natl Acad Sci U S A 105, 13580-13585. Khelaifia, S. & Raoult, D. (2016). Haloferax massiliensis sp. nov., the first human-associated halophilic archaea. New Microbes New Infect 12, 96-98. Lagier, J. C., Khelaifia, S., Alou, M. T., Ndongo, S., Dione, N., Hugon, P., Caputo, A., Cadoret, F., Traore, S. I., Seck, E. H., Dubourg, G., Durand, G., Mourembou, G., Guilhot, E., Togo, A., Bellali, S., Bachar, D., Cassir, N., Bittar, F., Delerce, J., Mailhe, M., Ricaboni, D., Bilen, M., Dangui Nieko, N. P., Dia Badiane, N. M., Valles, C., Mouelhi, D., Diop, K., Million, M., Musso, D., Abrahão, J., Azhar, E. I., Bibi, F., Yasir, M., Diallo, A., Sokhna, C., Djossou, F., Vitton, V., Robert, C., Rolain, J. M., La Scola, B., Fournier, P. E., Levasseur, A. & Raoult, D. (2016). Culture of previously uncultured members of the human gut microbiota by culturomics. Nat Microbiol 1, 16203. Levitt, M. D., Furne, J. K., Kuskowski, M. & Ruddy, J. (2006). Stability of human methanogenic flora over 35 years and a review of insights obtained from breath methane measurements. Clin Gastroenterol Hepatol 4, 123-129. Mihajlovski, A., Doré, J., Levenez, F., Alric, M. & Brugère, J. F. (2010). Molecular evaluation of the human gut methanogenic archaeal microbiota reveals an age-associated increase of the diversity. Environ Microbiol Rep 2, 272-280. Miller, T. L. & Wolin, M. J. (1982). Enumeration of Methanobrevibacter smithii in human feces. Arch Microbiol 131, 14-18. Oxley, A. P., Lanfranconi, M. P., Würdemann, D., Ott, S., Schreiber, S., McGenity, T. J., Timmis, K. N. & Nogales, B. (2010). Halophilic archaea in the human intestinal mucosa. Environ Microbiol 12, 2398-2410. Samuel, B. S., Hansen, E. E., Manchester, J. K., Coutinho, P. M., Henrissat, B., Fulton, R., Latreille, P., Kim, K., Wilson, R. K. & Gordon, J. I. (2007). Genomic and metabolic adaptations of Methanobrevibacter smithii to the human gut. Proc Natl Acad Sci U S A 104, 10643-10648. Söllinger, A., Schwab, C., Weinmaier, T., Loy, A., Tveit, A. T., Schleper, C. & Urich, T. (2016). Phylogenetic and genomic analysis of Methanomassiliicoccales in wetlands and animal intestinal tracts reveals clade-specific habitat preferences. FEMS Microbiol Ecol doi:10.1093/femsec/fiv149. Vanderhaeghen, S., Lacroix, C. & Schwab, C. (2015). Methanogen communities in stools of humans of different age and health status and co-occurrence with bacteria. FEMS Microbiol Lett 362, fnv092. Bacteria are prokaryotes, i.e. single-celled organisms that lack a nucleus and specialized organelles. They can be broadly classified into two large groups based on their Gram stain (Figure 1), and come in various shapes and sizes (Figure 2). 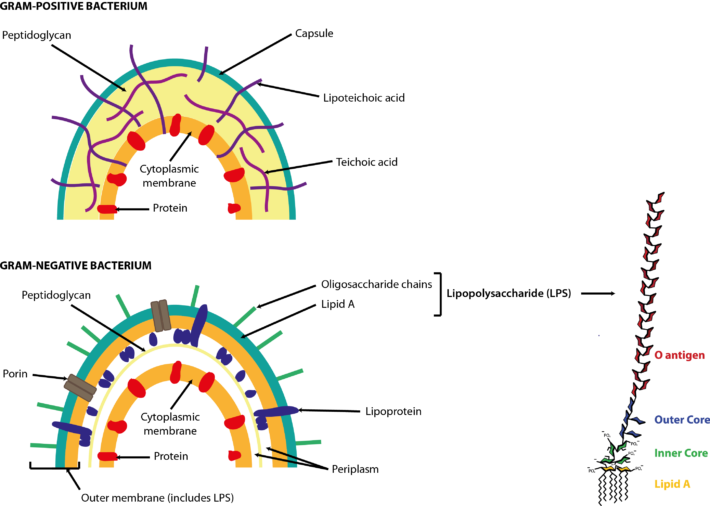 Figure 1. Cell walls of Gram-positive and Gram-negative bacteria. Both types of bacteria can be encapsulated or unencapsulated. Lipopolysaccharide (LPS) on the cell wall of Gram-negative bacteria is considered an endotoxin, and circulating levels of it within the body are associated with metabolic endotoxaemia and inflammation (Cani et al., 2008). The image of the detailed structure of LPS (by Mike Jones) was taken from Wikipedia and is available under a Creative Commons Attribution-Share Alike 3.0 Unported license, via Wikimedia Commons. Over 1000 different species of bacteria have been isolated from the human gastrointestinal tract, with bacteria belonging to the phyla Actinobacteria, Bacteroidetes, Firmicutes and Proteobacteria most predominant and diverse in the faeces of healthy adults (Rajilić-Stojanović et al., 2014; Browne et al., 2016; Lagier et al., 2016). They are by far the most abundant organisms inhabiting the human gastrointestinal tract; consequently, most gut microbiota studies focus on the characterization of bacteria. The gut bacterial microbiota has been referred to recently as the bacteriome, though this should not be confused with the insect bacteriome, a specialized organ that hosts endosymbiotic bacteria. 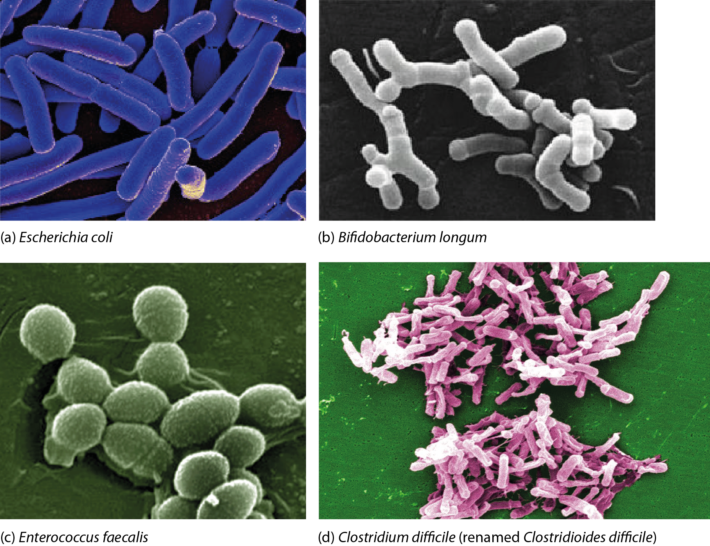 FIgure 2. Electron micrographs of bacteria commonly associated with the human gut microbiota showing some of the shapes of different bacteria. Other examples can be found in the NIAID and Sanofi Pasteur photo albums from which (a) and (d), respectively, were taken. Note that Clostridium difficile has been renamed ‘Clostridioides difficile‘ (Lawson et al., 2016). (b) By Julie6301, available under a Creative Commons Attribution-Share Alike 3.0 Unported license, via Wikimedia Commons. (c) Available under a Creative Commons Attribution-Share Alike 3.0 Unported license, via Wikimedia Commons. Photo credit: Janice Haney Carr Content Providers(s): CDC/ Pete Wardell derivative work: F. Lamiot. References Browne, H. P., Forster, S. C., Anonye, B. O., Kumar, N., Neville, B. A., Stares, M. D., Goulding, D. & Lawley, T. D. (2016). Culturing of ‘unculturable’ human microbiota reveals novel taxa and extensive sporulation. Nature 533, 543-546. Cani, P. D., Bibiloni, R., Knauf, C., Waget, A., Neyrinck, A. M., Delzenne, N. M. & Burcelin, R. (2008). Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 57, 1470-1481. Lagier, J. C., Khelaifia, S., Alou, M. T., Ndongo, S., Dione, N., Hugon, P., Caputo, A., Cadoret, F., Traore, S. I., Seck, E. H., Dubourg, G., Durand, G., Mourembou, G., Guilhot, E., Togo, A., Bellali, S., Bachar, D., Cassir, N., Bittar, F., Delerce, J., Mailhe, M., Ricaboni, D., Bilen, M., Dangui Nieko, N. P., Dia Badiane, N. M., Valles, C., Mouelhi, D., Diop, K., Million, M., Musso, D., Abrahão, J., Azhar, E. I., Bibi, F., Yasir, M., Diallo, A., Sokhna, C., Djossou, F., Vitton, V., Robert, C., Rolain, J. M., La Scola, B., Fournier, P. E., Levasseur, A. & Raoult, D. (2016). Culture of previously uncultured members of the human gut microbiota by culturomics. Nat Microbiol 1, 16203. Lawson, P. A., Citron, D. M., Tyrrell, K. L. & Finegold, S. M. (2016). Reclassification of Clostridium difficile as Clostridioides difficile (Hall and O’Toole 1935) Prévot 1938. Anaerobe 40, 95-99. Rajilić-Stojanović, M. & de Vos, W. M. (2014). The first 1000 cultured species of the human gastrointestinal microbiota. FEMS Microbiol Rev 38, 996-1047. It is important to recognize the difference between the microbiota and the microbiome. The microbiota refers to the micro-organisms and viruses associated with the human gastrointestinal tract. The microbiome refers to the genetic make-up of the whole of the microbiota: i.e. the genes from all the bacteria, eukaryotes, archaea and viruses. Metagenomic studies have focussed on the bacterial/archaeal and viral components of the microbiome (e.g. Breitbart et al., 2003; Qin et al., 2010; Reyes et al., 2010; Minot et al., 2011; Karlsson et al., 2012, 2013; Cotillard et al., 2013), but we also know the human faecal microbiome contains DNA from protozoa, helminths (worms) and fungi (unpublished data). The human gut microbiota comprises the bacteria, archaea, viruses, protozoa, helminths and fungi that inhabit your gastrointestinal tract. It was once thought there were 10 times the number of micro-organisms in your gastrointestinal tract than there were human cells in your entire body, with at least 10 times more viruses present than bacteria (Ley et al., 2006; Hoyles et al., 2014). However, a recent study has shown there are as many microbial cells present in the gut as there are human cells in the body (3.8×1013 for a 70 kg “reference man”), weighing approximately 200 g (Sender et al., 2016). Archaea and eukaryotes are also represented, but in much lower numbers than the bacteria or viruses. Although the number of microbial cells in the microbiota equals that of human cells, the bacterial/archaeal/viral component of the microbiome of the gut (faecal) microbiota of each individual has more than 10 times the number of genes of the human genome (Qin et al., 2010). The human gut microbiota lives in symbiosis with its host – i.e. you – providing, for example, essential non-nutrient factors, such as vitamins, butyrate (the preferred energy source of the colonic epithelium) from fermentation of dietary carbohydrates, protection against pathogens by out-competing them for nutrients and a substantial increase in the host’s ability to harvest nutrients from food (Roberfroid et al., 2010; Maynard et al., 2012). In turn, you provide the microbiota with a warm, nutrient-rich environment in which it can establish a relatively stable ecosystem (Maynard et al., 2012). Disturbances to the composition and/or diversity of the microbiota have been associated with numerous conditions (including obesity, type 2 diabetes, irritable bowel syndrome, colon cancer, metabolic syndrome, autism, liver disease and inflammatory bowel diseases). Different regions of the gastrointestinal tract have their own microbiotas, with the microbiotas’ composition in each of these regions dictated by a number of intrinsic and extrinsic factors. Intrinsic factors influencing the composition of the microbiota include gastrointestinal motility (peristalsis) and secretions, your age and health status, mucus, antimicrobial peptides, secretory immunoglobulin A, and oxygen and pH conditions (Figure 1); extrinsic factors include your diet (including probiotics and prebiotics) and any medications you are taking (including proton pump inhibitors, antacids, non-steroidal anti-inflammatory and prokinetics drugs, opioids, laxatives and antibiotics) (Simrén et al., 2013). By far the best-studied microbes are the bacteria, especially the faecal bacteria, though interest in isolating and characterizing the viruses that inhabit the gastrointestinal tract is growing. 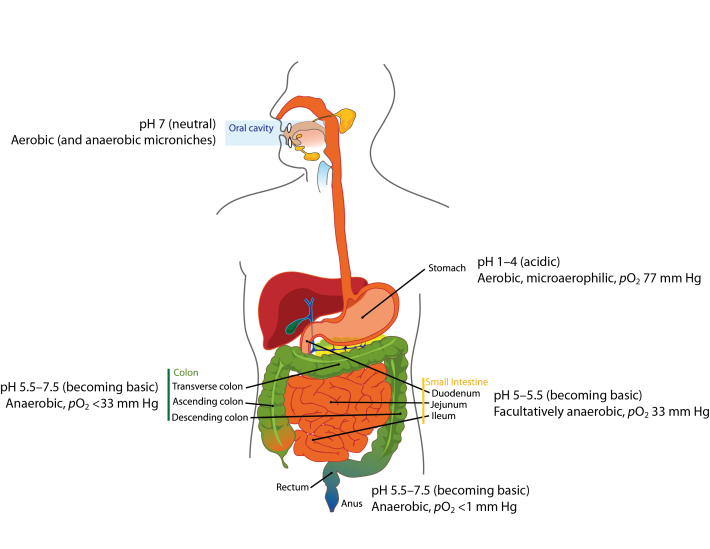 Figure 1. Oxygen and pH conditions throughout the digestive tract. The oral microbiota comprises predominantly aerobes, though within the mouth there are niches inhabited by anaerobic bacteria. As you move along the gastrointestinal tract, the atmosphere becomes more anaerobic and the pH more basic. Oxygen values taken from Espey (2013). Image from Wikimedia Commons, and modified from that released into the public domain by Mariana Ruiz Villarreal. References Breitbart, M., Hewson, I., Felts, B., Mahaffy, J. M., Nulton, J., Salamon, P. & Rohwer, F. (2003). Metagenomic analyses of an uncultured viral community from human feces. J Bacteriol 185, 6220-6223. Cotillard, A., Kennedy, S. P., Kong, L. C., Prifti, E., Pons, N., Le Chatelier, E., Almeida, M.,Quinquis, B., Levenez, F., Galleron, N., Gougis, S., Rizkalla, S., Batto, J. M., Renault, P., ANR MicroObes consortium, Doré, J., Zucker, J. D., Clément, K. & Ehrlich, S. D. (2013). Dietary intervention impact on gut microbial gene richness. Nature 500, 585-588. Espey, M. G. (2013). Role of oxygen gradients in shaping redox relationships between the human intestine and its microbiota. Free Radic Biol Med 55, 130–140. Hoyles, L., McCartney, A. L., Neve, H., Gibson, G. R., Sanderson, J. D., Heller, K. J. & van Sinderen, D. (2014). Characterization of virus-like particles associated with the human faecal and caecal microbiota. Res Microbiol 165, 803-812. Karlsson, F. H., Fåk, F., Nookaew, I., Tremaroli, V., Fagerberg, B., Petranovic, D., Bäckhed, F. & Nielsen, J. (2012). Symptomatic atherosclerosis is associated with an altered gut metagenome. Nat Commun 3, 1245. Karlsson, F. H., Tremaroli, V., Nookaew, I., Bergström, G., Behre, C. J., Fagerberg, B., Nielsen, J. & Bäckhed, F. (2013). Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature 498, 99-103. Ley, R. E., Peterson, D. A. & Gordon, J. I. (2006). Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell 124, 837-848. Maynard, C. L., Elson, C. O., Hatton, R. D. & Weaver, C. T. (2012). Reciprocal interactions of the intestinal microbiota and immune system. Nature 489, 231-241. Minot, S., Sinha, R., Chen, J., Li, H., Keilbaugh, S. A., Wu, G. D., Lewis, J. D. & Bushman, F. D. (2011). The human gut virome: inter-individual variation and dynamic response to diet. Genome Res 21, 1616-1625. Qin, J., Li, R., Raes, J., Arumugam, M., Burgdorf, K. S., Manichanh, C., Nielsen, T., Pons, N., Levenez, F., Yamada, T., Mende, D. R., Li, J., Xu, J., Li, S., Li, D., Cao, J., Wang, B., Liang, H., Zheng, H., Xie, Y., Tap, J., Lepage, P., Bertalan, M., Batto, J. M., Hansen, T., Le Paslier, D., Linneberg, A., Nielsen, H. B., Pelletier, E., Renault, P., Sicheritz-Ponten, T., Turner, K., Zhu, H., Yu, C., Li, S., Jian, M., Zhou, Y., Li, Y., Zhang, X., Li, S., Qin, N., Yang, H., Wang, J., Brunak, S., Doré, J., Guarner, F., Kristiansen, K., Pedersen, O., Parkhill, J., Weissenbach, J., MetaHIT Consortium, Bork, P., Ehrlich, S. D. & Wang, J. (2010). A human gut microbial gene catalogue established by metagenomic sequencing. Nature 464, 59-65. Reyes, A., Haynes, M., Hanson, N., Angly, F. E., Heath, A. C., Rohwer, F. & Gordon, J. I. (2010). Viruses in the faecal microbiota of monozygotic twins and their mothers. Nature 466, 334-338. Roberfroid, M., Gibson, G. R., Hoyles, L., McCartney, A. L., Rastall, R., Rowland, I., Wolvers, D., Watzl, B., Szajewska, H., Stahl, B., Guarner, F., Respondek, F., Whelan, K., Coxam, V., Davicco, M. J., Léotoing, L., Wittrant, Y., Delzenne, N. M., Cani, P. D., Neyrinck, A. M. & Meheust, A. (2010). Prebiotic effects: metabolic and health benefits. Br J Nutr 104 Suppl 2, S1-S63. Sender, R., Fuchs, S. & Milo, R. (2016). Revised estimates for the number of human and bacteria cells in the body. PLoS Biol 14, e1002533. Simrén, M., Barbara, G., Flint, H. J., Spiegel, B. M., Spiller, R. C., Vanner, S., Verdu, E. F., Whorwell, P. J., Zoetendal, E. G. & Rome Foundation Committee (2012). Intestinal microbiota in functional bowel disorders: a Rome foundation report. Gut 62, 159-176. The upper digestive tract has several innate defences to protect you from your own secretions and from microbial invaders. These defences include mucus and gastric acid, enterosalivary nitrate circulation, peristalsis and lymphoid tissue in the form of Peyer’s patches. Mucus, a bicarbonate-rich slimy substance secreted by superficial epithelial cells throughout your stomach, protects you from foreign microbes, and protects you from your gastric acid. In general, bacteria and other microbes that you ingest are destroyed by their exposure to gastric (stomach) acid (Shaffer, 1997; Holzapfel et al., 1998; Dunne et al., 2001; Guarner & Malagelada, 2003). For this reason, the stomach is viewed as the main barrier against entry of foreign microbes into the gastrointestinal tract. However, it is important to note that, even with its acidic environment, the stomach is not sterile.
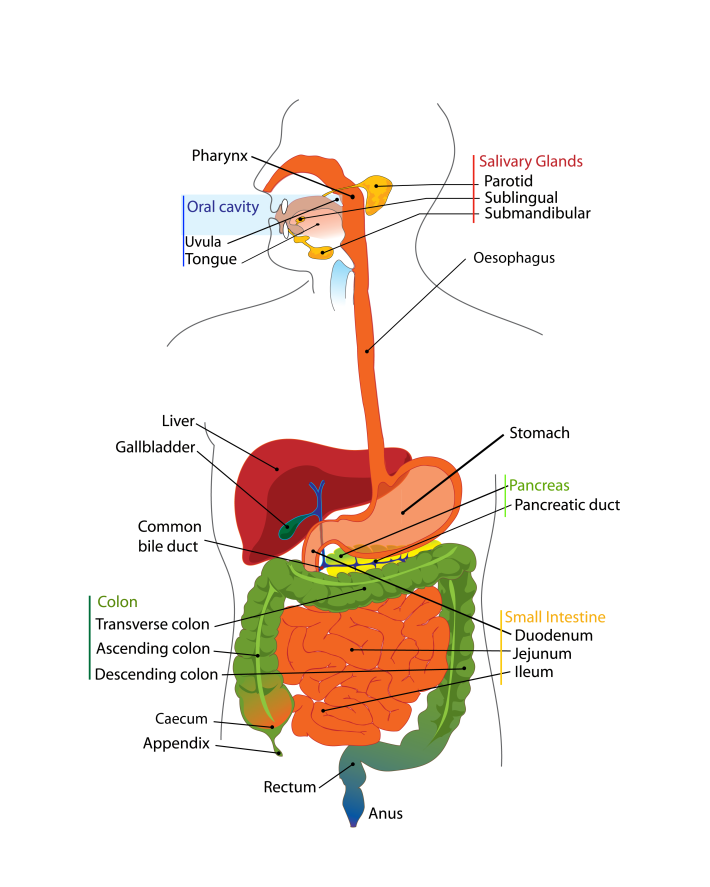
Not chewing your food properly leads to reduced peristalsis and reduced production of gastric acid and saliva (O’May et al., 2005a, b). Inorganic nitrate ingested as part of the diet is absorbed in the stomach and ~25 % is concentrated in the salivary glands and re-secreted into the mouth (Deri et al., 2005). Around 30 % (~0.3 mM) of this nitrate is reduced to nitrite by the oral microbiota on the tongue (O’May et al., 2005a, b; Deri et al., 2005). Acidification of the nitrite in the stomach generates nitric oxide: at the gastric level, this molecule has antibacterial properties, and increases gastric motility, mucus production and mucosal blood flow (Deri et al., 2005). Breakdown of the aforementioned defence mechanisms leads to bacterial overgrowth in the upper gastrointestinal tract (stomach and duodenum) (O’May et al., 2005a, b), with the degree of overgrowth dependent upon the elevation of the gastric pH (Kerckhoffs et al., 2006). In addition to the numerous defence mechanisms associated with the stomach, protection of the rest of the digestive tract from foreign microbes comes in the form of hepatic and pancreatic secretions and secretions from the small intestine. Bile acids are synthesized (500–700 ml per day) by the liver from cholesterol and secreted from the liver or gallbladder into the duodenum in the conjugated form (Dunne et al., 2001). These bile acids are chemically modified (i.e. deconjugated, dehydroxylated, dehydrogenated or deglucuronidated) by gut bacteria. Both conjugated and deconjugated forms of bile acids have been shown to have antibacterial properties, with the deconjugated forms being more inhibitory and Gram-positive bacteria more sensitive than Gram-negative bacteria (Dunne et al., 2001). The difference in sensitivity between Gram-positive and Gram-negative bacteria is largely due to differences in the composition of their cell walls. An intact ileocaecal valve is also an important barrier to backflow of colonic bacteria into the ileum (Kerckhoffs et al., 2006). References Deri, L., Pietraforte, D., Scorza, G., Napolitano, A., Fogliano, V. & Minetti, M. (2005). Apples increase nitric oxide production by human saliva at the acidic pH of the stomach: a new biological function for polyphenols with a catechol group? Free Rad Biol Med 39, 668–681. Dunne, C., O’Mahony, L., Murphy, L. & 11 other authors (2001). In vitro selection criteria for probiotic bacteria of human origin: correlation with in vivo findings. Am J Clin Nutr 73 (suppl.), 386S–392S. Guarner, F. & Malagelada, J.-R. (2003). Gut flora in health and disease. Lancet 361, 512–519. Holzapfel, W. H., Haberer, P., Snel, J., Schillinger, U. & Huis in’t Veld, J. H. J. (1998). Overview of gut flora and probiotics. Int J Food Microbiol 41, 85–101. Kerckhoffs, A. P. M., Samson, M., van Berge Henegouwen, G. P., Akkermans, L. M. A., Nieuwenhuijs, V. B. & Visser, M. R. (2006). Sampling microbiota in the human gastrointestinal tract. In Gastrointestinal Microbiology, pp. 25–50. Edited by A. Ouwehand & E. E. Vaughan. New York: Taylor & Francis Ltd. O’May, G. A., Reynolds, N., Smith, A. R., Kennedy, A. & Macfarlane, G. T. (2005a). Effect of pH and antibiotics on microbial overgrowth in the stomachs and duodena of patients undergoing percutaneous endoscopic gastromy feeding. Appl Environ Microbiol 71, 3059–3065. O’May, G. A., Reynolds, N. & Macfarlane, G. T. (2005b). Effect of pH on an in vitro model of gastric microbiota in enteral nutrition patients. Appl Environ Microbiol 71, 4777–4783. Shaffer, E. A. (1997). Digestive system, physiology and biochemistry. In Encyclopedia of Human Biology, 2nd edn, vol. 3, pp. 343–354. Edited by R. Dulbecco. San Diego, CA: Academic Press. Do you think about what happens to food after you have eaten it? Probably not, but to understand how microbes contributing to the gut microbiota survive inside you, it is important to have a basic understanding of how the human digestive tract works. The following won’t go into details as to how the organs of your digestive tract work together to convert food to energy and nutrients to keep your body fuelled and healthy. Instead, it will concentrate on how the nutrients in your food are made available to the microbes that inhabit every inch of your digestive tract. The easiest way to think about your digestive tract is to picture it as a tube that runs through your body, from your mouth to your anus (Figure 1). If it were not for the valves and sphincters that are found throughout your digestive tract, your body would essentially be a hollow tube. Valves ensure the food you eat goes in only one direction of travel, while sphincters are rings of muscle that surround and protect the opening or closing parts of your digestive tract, such as your stomach or anus. Your whole digestive tract is about 9 metres long. What we refer to as the gastrointestinal tract includes the regions of the digestive tract between the oesophagus and the anus. Activities of and/or secretions from the liver, gallbladder and pancreas aid the digestive process, and these organs are considered part of the digestive tract. There is a strong association between the activities of gut microbes and the healthy functioning of the liver and other organs of the body. Saliva You take food into your mouth and chew it to produce a bolus (a ball of chewed food). The majority of the food you eat comprises carbohydrates, fats, protein and water, with varying small amounts of vitamins, minerals, nitrates, nucleotides, methylamines and additives. The action of chewing causes saliva to be secreted from your salivary glands into your mouth. Saliva has a number of functions that aid in the digestion of food and in protecting you from harmful bacteria (Valdez & Fox, 1991; Abiko & Saitoh, 2007). It lubricates the bolus so that it passes down your oesophagus easily, with this smooth passage also protecting the lining of your mouth and oesophagus from wear and tear. The enzymes salivary amylase and lingual lipase start to break down starch and fats in the bolus to simpler sugars and less-complex fats, respectively (Valdez & Fox, 1991; Phan & Tso, 2001). These simple fats and sugars (and undigestible carbohydrates) pass into your stomach, along with proteins. After you swallow the bolus, peristalsis (a wave-like motion caused by the rhythmic contraction and relaxation of the smooth muscles that run along your oesophagus) propels the bolus down your oesophagus to your stomach. The stomach Your stomach is essentially a short-term storage reservoir for food and contains gastric acid, with a pH between 1 and 4 due to hydrochloric acid secreted by parietal cells lining the stomach (Encyclopaedia Britannica). In addition to hydrochloric acid, gastric acid comprises sodium chloride and potassium chloride. The acidity of the stomach serves three main roles: i) aiding in the digestion of food particles, ii) protecting you from potentially dangerous foreign microbes (by foreign microbes I mean organisms that are either not normal members of the gut microbiota or normally present at very low levels), and iii) aiding in the digestion of proteins. Acidity of the stomach environment activates pepsinogen to the digestive enzyme pepsin. It also aids in unravelling (denaturing) proteins so that they are more easily broken down to their constituent amino acids by enzymes such as pepsin. Gastric lipase aids in the digestion of dietary lipids. Vigorous contractions of gastric smooth muscle mix and grind foodstuffs with gastric secretions, resulting in the formation of chyme, a thick, semi-liquid mass of partially digested food. The small intestine The small intestine (comprising the duodenum, jejunum and ileum) is a long, winding tube that is ~3 metres long and 2–3 cm in diameter (Espey, 2013). It is the longest organ in your body. Food, in the form of chyme, passes from the stomach through the pyloric sphincter to the duodenum. The acidity of the chyme is reduced by bile acids secreted from the gallbladder, and enzymes secreted from the pancreas and duodenal cells help digest components of the chyme. For example, pancreatic amylase continues the break down of digestible carbohydrates to simple sugars, while trypsin and chymotrypsin (secreted from the pancreas in the inactive forms trypsinogen and chymotrypsinogen, respectively) break down proteins to their constituent amino acids. Peristalsis pushes chyme along the small intestine. The surface area of the small intestine is large compared with its length due to its villi and ‘brush border’, which comprises thousands of finger-like projections called microvilli (Figure 2). Consequently, the small intestine is the primary site within the human gastrointestinal tract for nutrient (e.g. glucose, amino acids, fatty acids), water and electrolyte (sodium, chloride, potassium) uptake into the blood. Up to 95 % of absorption of nutrients occurs in the small intestine. 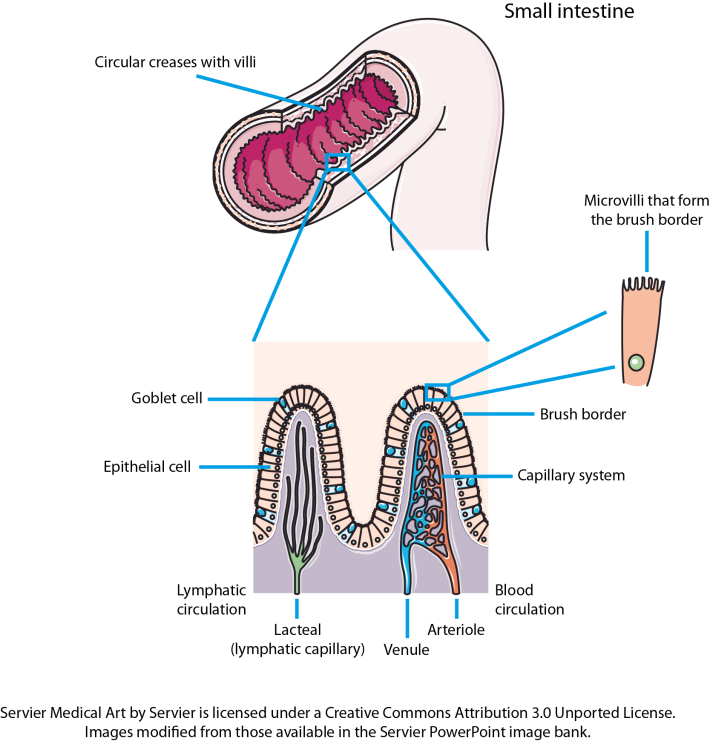 Figure 2. The small intestine is convoluted, with the folds forming thousands of villi that project into the intestinal lumen. There are 20–30 villi per square millimetre of small-intestinal tissue (Espey, 2013). Each of the epithelial cells covering the villi is covered with microvilli that form the brush border of the small intestine. The huge surface area created by the villi and the microvilli increases the amount of nutrients that can be taken up by the small intestine. Goblet cells secrete mucus into the intestinal lumen, lubricating the small intestine. Other cells present, but not shown on the image, include enteroendocrine and stem cells. The caecum, appendix and large intestine (colon)The caecum is a pouch that connects the ileum to the proximal colon, and is the beginning of the large intestine. It receives undigested dietary material from the ileum, via the ileocaecal valve. The caecum is connected to the appendix and is separated from the contents of the ascending colon by the caecocolic junction. In herbivores, undigested dietary material that reaches the caecum is fermented by the caecal microbiota. The human caecal microbiota is capable of fermenting dietary carbohydrates (Flourie et al., 1986), but the contribution this makes to gut fermentation of dietary substances that escape digestion in the upper gastrointestinal tract is minimal when you consider the contribution of the colonic microbiota to this process. Consequently, the human caecum is considered a dead-end pouch that connects the upper and lower intestinal tract. However, it is likely that the caecal microbiota is important in establishing/maintaining the appendix and colonic microbiota, and may play a role in human health. The appendix is a 5–10 cm long, 0.1–0.5 cm wide pouch that is an extension of the caecum (Randal Bollinger et al., 2007). It does not play a role in digestion. Instead, it is part of the immune system, and acts as a ‘safe house’ for the commensal microbiota that can re-inoculate the colonic microbiota after a bout of diarrhoea (Laurin et al., 2011). In humans, the large intestine serves as the main site of microbial fermentation of dietary substrates that escape digestion in the upper gastrointestinal tract. The large intestine is also involved in the uptake of water from digesta and waste material prior to the expulsion of faeces from the body (defecation). References Abiko, Y. & Saitoh, M. (2007). Salivary defensins and their importance in oral health and disease. Curr Pharm Des 13, 3065–3072. Encyclopaedia Britannica: Human Digestive System – proteins. Espey, M. G. (2013). Role of oxygen gradients in shaping redox relationships between the human intestine and its microbiota. Free Radic Biol Med 55, 130–40. Flourie, B., Florent, C,, Jouany, J. P., Thivend, P., Etanchaud, F. & Rambaud, J. C. (1986). Colonic metabolism of wheat starch in healthy humans. Effects on fecal outputs and clinical symptoms. Gastroenterology 90, 111–119. Laurin, M., Everett, M. L. & Parker, W. (2011). The cecal appendix: one more immune component with a function disturbed by post-industrial culture. Anat Rec (Hoboken) 294, 567–579. Phan, C. T. & Tso, P. (2001).Intestinal lipid absorption and transport. Front Biosci 6, D299–D319. Randal Bollinger, R., Barbas, A. S., Bush, E. L., Lin, S. S. & Parker, W. (2007). Biofilms in the large bowel suggest an apparent function of the human vermiform appendix. J Theor Biol 249, 826–831. Valdez & Fox (1991).Interactions of the salivary and gastrointestinal systems I. The role of saliva in digestion. Dig Dis 9, 125–132. This older website, run by Colorado State University, is very good for more-detailed information on the human digestive system. |
Lesley HoylesProfessor of Microbiome and Systems Biology, Nottingham Trent University ArchivesCategories
All
|