|
It is important to recognize the difference between the microbiota and the microbiome. The microbiota refers to the micro-organisms and viruses associated with the human gastrointestinal tract. The microbiome refers to the genetic make-up of the whole of the microbiota: i.e. the genes from all the bacteria, eukaryotes, archaea and viruses. Metagenomic studies have focussed on the bacterial/archaeal and viral components of the microbiome (e.g. Breitbart et al., 2003; Qin et al., 2010; Reyes et al., 2010; Minot et al., 2011; Karlsson et al., 2012, 2013; Cotillard et al., 2013), but we also know the human faecal microbiome contains DNA from protozoa, helminths (worms) and fungi (unpublished data). The human gut microbiota comprises the bacteria, archaea, viruses, protozoa, helminths and fungi that inhabit your gastrointestinal tract. It was once thought there were 10 times the number of micro-organisms in your gastrointestinal tract than there were human cells in your entire body, with at least 10 times more viruses present than bacteria (Ley et al., 2006; Hoyles et al., 2014). However, a recent study has shown there are as many microbial cells present in the gut as there are human cells in the body (3.8×1013 for a 70 kg “reference man”), weighing approximately 200 g (Sender et al., 2016). Archaea and eukaryotes are also represented, but in much lower numbers than the bacteria or viruses. Although the number of microbial cells in the microbiota equals that of human cells, the bacterial/archaeal/viral component of the microbiome of the gut (faecal) microbiota of each individual has more than 10 times the number of genes of the human genome (Qin et al., 2010). The human gut microbiota lives in symbiosis with its host – i.e. you – providing, for example, essential non-nutrient factors, such as vitamins, butyrate (the preferred energy source of the colonic epithelium) from fermentation of dietary carbohydrates, protection against pathogens by out-competing them for nutrients and a substantial increase in the host’s ability to harvest nutrients from food (Roberfroid et al., 2010; Maynard et al., 2012). In turn, you provide the microbiota with a warm, nutrient-rich environment in which it can establish a relatively stable ecosystem (Maynard et al., 2012). Disturbances to the composition and/or diversity of the microbiota have been associated with numerous conditions (including obesity, type 2 diabetes, irritable bowel syndrome, colon cancer, metabolic syndrome, autism, liver disease and inflammatory bowel diseases). Different regions of the gastrointestinal tract have their own microbiotas, with the microbiotas’ composition in each of these regions dictated by a number of intrinsic and extrinsic factors. Intrinsic factors influencing the composition of the microbiota include gastrointestinal motility (peristalsis) and secretions, your age and health status, mucus, antimicrobial peptides, secretory immunoglobulin A, and oxygen and pH conditions (Figure 1); extrinsic factors include your diet (including probiotics and prebiotics) and any medications you are taking (including proton pump inhibitors, antacids, non-steroidal anti-inflammatory and prokinetics drugs, opioids, laxatives and antibiotics) (Simrén et al., 2013). By far the best-studied microbes are the bacteria, especially the faecal bacteria, though interest in isolating and characterizing the viruses that inhabit the gastrointestinal tract is growing. 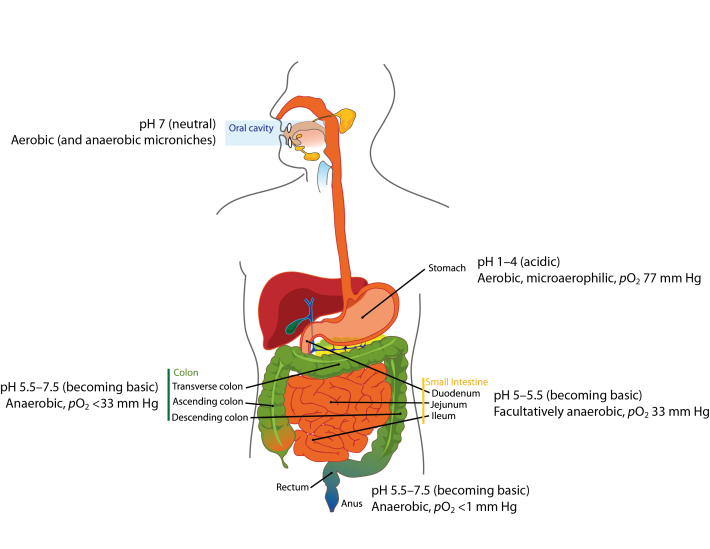 Figure 1. Oxygen and pH conditions throughout the digestive tract. The oral microbiota comprises predominantly aerobes, though within the mouth there are niches inhabited by anaerobic bacteria. As you move along the gastrointestinal tract, the atmosphere becomes more anaerobic and the pH more basic. Oxygen values taken from Espey (2013). Image from Wikimedia Commons, and modified from that released into the public domain by Mariana Ruiz Villarreal. References Breitbart, M., Hewson, I., Felts, B., Mahaffy, J. M., Nulton, J., Salamon, P. & Rohwer, F. (2003). Metagenomic analyses of an uncultured viral community from human feces. J Bacteriol 185, 6220-6223. Cotillard, A., Kennedy, S. P., Kong, L. C., Prifti, E., Pons, N., Le Chatelier, E., Almeida, M.,Quinquis, B., Levenez, F., Galleron, N., Gougis, S., Rizkalla, S., Batto, J. M., Renault, P., ANR MicroObes consortium, Doré, J., Zucker, J. D., Clément, K. & Ehrlich, S. D. (2013). Dietary intervention impact on gut microbial gene richness. Nature 500, 585-588. Espey, M. G. (2013). Role of oxygen gradients in shaping redox relationships between the human intestine and its microbiota. Free Radic Biol Med 55, 130–140. Hoyles, L., McCartney, A. L., Neve, H., Gibson, G. R., Sanderson, J. D., Heller, K. J. & van Sinderen, D. (2014). Characterization of virus-like particles associated with the human faecal and caecal microbiota. Res Microbiol 165, 803-812. Karlsson, F. H., Fåk, F., Nookaew, I., Tremaroli, V., Fagerberg, B., Petranovic, D., Bäckhed, F. & Nielsen, J. (2012). Symptomatic atherosclerosis is associated with an altered gut metagenome. Nat Commun 3, 1245. Karlsson, F. H., Tremaroli, V., Nookaew, I., Bergström, G., Behre, C. J., Fagerberg, B., Nielsen, J. & Bäckhed, F. (2013). Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature 498, 99-103. Ley, R. E., Peterson, D. A. & Gordon, J. I. (2006). Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell 124, 837-848. Maynard, C. L., Elson, C. O., Hatton, R. D. & Weaver, C. T. (2012). Reciprocal interactions of the intestinal microbiota and immune system. Nature 489, 231-241. Minot, S., Sinha, R., Chen, J., Li, H., Keilbaugh, S. A., Wu, G. D., Lewis, J. D. & Bushman, F. D. (2011). The human gut virome: inter-individual variation and dynamic response to diet. Genome Res 21, 1616-1625. Qin, J., Li, R., Raes, J., Arumugam, M., Burgdorf, K. S., Manichanh, C., Nielsen, T., Pons, N., Levenez, F., Yamada, T., Mende, D. R., Li, J., Xu, J., Li, S., Li, D., Cao, J., Wang, B., Liang, H., Zheng, H., Xie, Y., Tap, J., Lepage, P., Bertalan, M., Batto, J. M., Hansen, T., Le Paslier, D., Linneberg, A., Nielsen, H. B., Pelletier, E., Renault, P., Sicheritz-Ponten, T., Turner, K., Zhu, H., Yu, C., Li, S., Jian, M., Zhou, Y., Li, Y., Zhang, X., Li, S., Qin, N., Yang, H., Wang, J., Brunak, S., Doré, J., Guarner, F., Kristiansen, K., Pedersen, O., Parkhill, J., Weissenbach, J., MetaHIT Consortium, Bork, P., Ehrlich, S. D. & Wang, J. (2010). A human gut microbial gene catalogue established by metagenomic sequencing. Nature 464, 59-65. Reyes, A., Haynes, M., Hanson, N., Angly, F. E., Heath, A. C., Rohwer, F. & Gordon, J. I. (2010). Viruses in the faecal microbiota of monozygotic twins and their mothers. Nature 466, 334-338. Roberfroid, M., Gibson, G. R., Hoyles, L., McCartney, A. L., Rastall, R., Rowland, I., Wolvers, D., Watzl, B., Szajewska, H., Stahl, B., Guarner, F., Respondek, F., Whelan, K., Coxam, V., Davicco, M. J., Léotoing, L., Wittrant, Y., Delzenne, N. M., Cani, P. D., Neyrinck, A. M. & Meheust, A. (2010). Prebiotic effects: metabolic and health benefits. Br J Nutr 104 Suppl 2, S1-S63. Sender, R., Fuchs, S. & Milo, R. (2016). Revised estimates for the number of human and bacteria cells in the body. PLoS Biol 14, e1002533. Simrén, M., Barbara, G., Flint, H. J., Spiegel, B. M., Spiller, R. C., Vanner, S., Verdu, E. F., Whorwell, P. J., Zoetendal, E. G. & Rome Foundation Committee (2012). Intestinal microbiota in functional bowel disorders: a Rome foundation report. Gut 62, 159-176. Comments are closed.
|
Lesley HoylesProfessor of Microbiome and Systems Biology, Nottingham Trent University ArchivesCategories
All
|
